Share4Rare toolkit for patient advocacy
Patient involvement in research
One of the most important areas in advocacy covers patients’ involvement (PI) in clinical research, since research cannot be the field that is driven by researcher’ interests alone, but it is developed through interaction and dialogue among all stakeholders. This is particularly relevant for Share4Rare, considering that the project will connect patients and researchers with the common goal of advancing research in rare disease.
Concretely, in the last ten years, there has been growing interest among patient organisations and funding agencies to involve patients in revising clinical research. Most of the demands for PI address both academic and industry clinical trials (testing new therapies), but also non-clinical research (e.g. discover new molecular targets; develop new agents or relevant biomarkers for different patients subgroups). Despite this becoming a common practice across Europe, a solid guidance to support the patient advocates or organisations has not yet been developed.

Photo by rawpixel on Unsplash
PI (Patient Involvement) has incontestable benefits for both patients and research, the most relevant being:
a) Making sure that research addresses patients’ real unmet needs and not professional perceptions of patients’ needs.
b) Eliminating ‘waste’ from research.
In their Lancet series, Chalmers and colleagues stated that as much of 85% of all clinical research was waste, with lacking early patient involvement as one of the reasons.
As such, patient involvement must start at the very early stages of medicines development - when research directions are set - and continue during the rest of the phases: research design, planning, execution, dissemination and post-approval activities (Geissler et al., 2017).
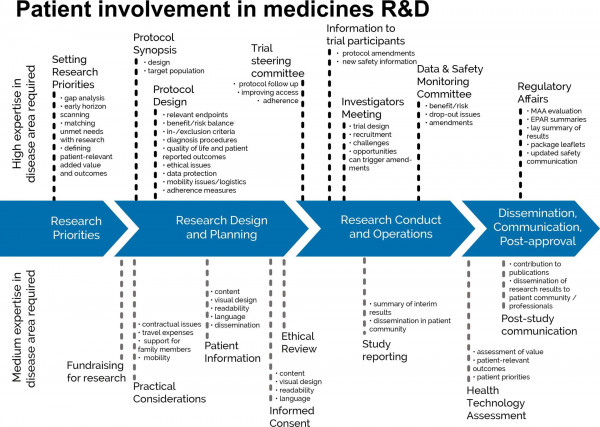
Reference: https://journals.sagepub.com/eprint/6J5ErcVqeCi4BDdCE7FD/full
At the same time, the treatment landscape of many diseases is subject to considerable progression. In this more demanding context the value and challenges of true patient involvement need to be correctly addressed (Ryll et al., 2017):
- Patients are an upcoming stakeholder group and, thanks to the internet, better informed and connected than ever before.
- With stakes as high as their own lives, patients are increasingly dissatisfied with a passive role in a research system failing to deliver much-needed results. They invest considerable resources to educate themselves to expert level; they collaborate to an unprecedented degree at minimal cost to effectively impose their views.
- In the last years, ‘patient-centricity’ has become a buzzword, but studying patients is not the same as studying what patients want and need. Tools need to be tested both for internal as well as external validity, to ensure that patient outcomes are not only reported but also relevant.
- The time and format of patient involvement is routinely defined by non-patients and is therefore limited by the concepts of non-patients. This is however changing and also patients in oncology are learning from the previous experiences of the HIV community.
- Patient involvement in research is not without challenges, because the methodology is in its infancy. However multi-stakeholder initiatives have started to provide guidance and tools to facilitate the process. Future research projects should provide structure, guidance and resources to ensure patient involvement is genuine. Relevant education is necessary.