
What is a patient registry?

Impact of neuromuscular diseases on education and working opportunities of patients and carers
A patient registry collects information about patients who are affected by a particular condition. Registries are databases containing quantitative and qualitative data about the patients.
In rare disease research, registries play an important role in the therapy development pathway. In fact, registries can:
- Identify participants for clinical trials.
- Help develop care standards, to help improve the care people receive.
- Support specific research questions.
- Provide information for doctors and scientists to learn more about rare diseases.
- Represent a link between patients and the research community, providing the opportunity for people to receive information directly relevant to their condition (for example, through newsletters).
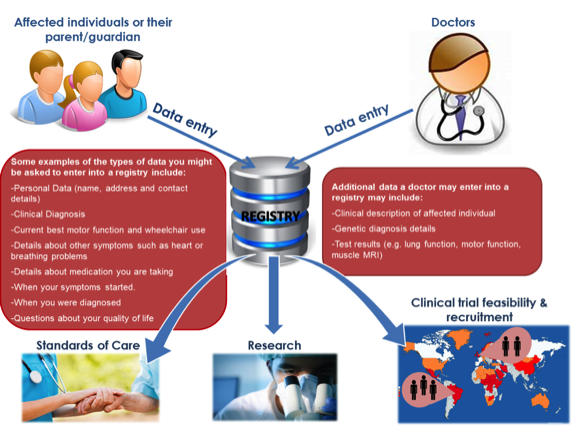
The data in registries can be entered by patients themselves, by their doctor or by a combination of the two. For example, TREAT-NMD can advise if a registry is available for a particular neuromuscular condition and a list of registries by disease can be found here.
Newcastle University Patient Registries
There are 7 registries managed from Newcastle University at the John Walton Muscular Dystrophy Centre:
-
The UK SMA Patient Registry.
- The Global FKRP Registry.
- The UK Myotonic Dystrophy Patient Registry.
- The UK FSHD Patient Registry.
- The MTM and CNM Patient Registry.
- The International GNE Myopathy Patient Registry.
- The Global Registry for COL6-Related Dystrophies.
Patient registries can be used in many ways. Below are a few examples in which the Newcastle registries have been used successfully:
- The UK DM Registry has previously been used to support the recruitment onto a phase II clinical trial of tideglusib in teenagers and adults with congenital and childhood-onset DM. The registry has also been used to support a falls and fall-associated fractures survey in patients with DM1.
- The UK FSHD Registry was used to help a pharmaceutical company gain patient insight into their upcoming clinical trial protocol. This was captured via a survey sent out through the registry.
- The UK SMA Registry was used to distribute an EU wide survey regarding patient quality of life and pain.
- The Global FKRP Registry was used to support participant recruitment onto a phase III clinical trial of deflazacort in adults with LGMD2I.
- The GNE Myopathy Registry was used to support participant recruitment onto a phase III clinical trial of aceneuramic acid in adults with GNE myopathy.
- Many of the registries have collectively supported participant recruitment onto a research project investigating activity monitoring in patients with neuromuscular conditions.
Patient registries can also be used as promotional and communications tools. Since 2019, the Newcastle University registries have:
- Been uploaded to Clinical trials.gov.
- Had some registry information has been updated on RD Connect and Orphanet.
- Begun the process of getting registries on ERDRI Database.
- Produced at least one newsletter.
- Created drug development pipeline charts for diseases like DM, DMD, FSHD, SMA, LGMD and MTM.
- Updated their respective registry information leaflets.
- Created a “why join a patient registry” leaflet that covers all seven registries.
- Begun the process of creating a communications plan so that patients receive regular research and registry updates at quarterly intervals for certain registries.
- Attended various national and international conferences where the registries were presented (poster and oral).
- Conducted extensive networking via LinkedIn and at conferences.
- Produced multiple publications involving the registries.
