
What is gene therapy? Basic concepts and current state of research
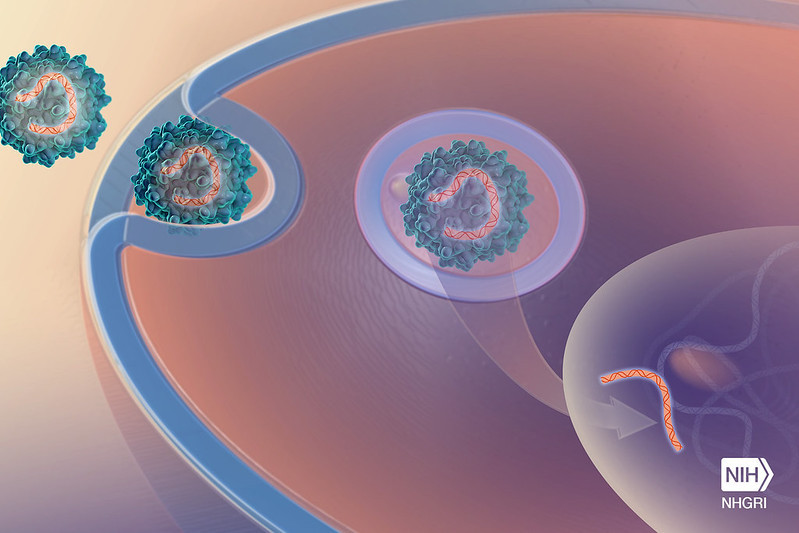
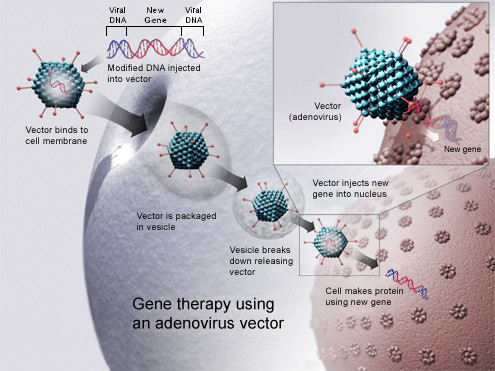
Gene therapy fixes genetic mutations that cause a pathology or the malfunction of a biological process by introducing a “correct” copy of the affected gene into our cells. We can achieve this in several ways, and its difficulty will depend on the mutated gene or genes, the type of mutation or the affected organs. Gene therapy can also turn off genes that are causing a health issue or introduce new genes that help the body fight the problem.
How gene therapy works
To introduce the gene or genes of interest into the body, we must use a "vehicle" to deliver the correct DNA fragment to the target cells. In most cases, this vehicle or vector, as scientists call it, is usually a genetically modified virus that is no longer able to infect. We insert the gene of interest into the modified virus and this virus, having the ability to transfer genetic material to human cells, will introduce it. In most cases, this procedure has proven to be very effective, and in recent decades, it has been explored for many diseases.
Gene therapy can be given in vivo or ex vivo. In the first case, the vector with the correct gene is introduced directly into the patient, usually by injecting it. In the case of ex vivo therapy, we extract blood, bone marrow, or other tissue from the patient, and transfer the desired gene into these cells in the laboratory, and then the healthy cells are injected into the patient. Ex vivo therapy is common in cases of leukaemia or sickle cell anaemia, as cancerous cells (in the first case) or malfunctioning cells (in the second case) are in the blood of the patients.
The main problem of gene therapy lies in the adverse effects caused by the vectors. Although over the years the safety of vectors has improved a lot, in some cases it is still necessary to investigate further to minimize the secondary effects derived from their use.
Where are we now?
When a news headline says that a regulatory agency has approved a therapy, it is important that we read the entire text of the news, because it can lead to confusion. Sometimes a treatment is approved for its experimental use in a clinical trial, and this does not mean that it has been approved for its commercial use. We must understand that many of the gene therapy treatments echoed by the media are usually still in the experimental phase, that is, they are still being tested on patients to certify their safety and efficacy and they are still passing from one study phase to another. For example, a treatment may be tested in cells (in a cell culture on the laboratory), pass this phase, and then scientists may analyse its properties in mice, or they may go from mice to clinical trials, from phase 1 to 2, etc. However, none of this means that we can use it to treat people on a large scale.
For example, a few months ago we talked on the blog about a therapy for epidermolysis bullosa applied directly on the skin. This therapy is not commercially available; it is still undergoing several clinical trials. We also recently discussed how a gene therapy for congenital muscular dystrophy had shown encouraging results, but we pointed out that it had been studied in patient cells.
In 2017, the FDA (the drug regulatory agency in the United States), approved the first gene therapy for commercial use, a treatment for acute lymphoblastic leukaemia. This summer, the agency approved a therapy for paediatric and adult patients with beta thalassemia, and a few months ago, two companies concluded negotiations to manufacture a treatment for Leber's congenital amaurosis, a hereditary retinal dystrophy that causes blindness and that had no therapeutic option to date. Today, there are thousands of similar therapies under investigation, waiting to hit the market as soon as they pass all the required procedures.
A relatively high price
The high costs of most of these therapies make it complicated for them to enter the market. Moreover, in some cases, companies can take advantage of having the only treatment available and charge an excessive price, although luckily there are legal mechanisms that regulate these practices. Despite the high cost of many gene therapies, experts argue that, as they are once-in-a-lifetime treatments, they would not be as expensive as current daily treatments in the long run.
In summary, gene therapy is showing promising results to treat many genetic diseases, but we still need more studies for most of them to finally make it through, not mentioning economic obstacles that block their availability. We must be patient while continuing to closely follow ongoing investigations because, in the future, they may be able to provide a real treatment for our disease.
