
Share4Rare criteria for meaningful patient involvement in clinical research

Patient involvement in medicines development continues to grow, as all stakeholders involved in developing accessible medicines realize they should be involving the end customer - the patient - from the very earliest stages of the research and development process. This is particularly pertinent within the rare disease community, as smaller patient populations need a much closer collaboration between researchers and people affected by disease.
However, patient involvement in the design of clinical research remains fragmented and inconsistent. Agreement on the importance of patient engagement in medicines development is becoming the norm across the healthcare ecosystem. But we are still far from agreeing on how to define qualitative patient engagement.
A major survey carried out by EURORDIS in 2018 found that more than 1/3 (37%) of rare disease patients had already participated in a research study. Among those who had participated in research, however, just 18% specified they had participated in research to develop treatments and therapies. This collaboration is also patchy geographically; the survey revealed that participation in research to develop treatments and therapies varies significantly across patients’ countries and disease area. Results also illustrated a gender gap in inclusion in clinical studies: more men (21%) than women (16%) had participated in research to develop treatments.
PEQG: A tool to assess patient engagement
That is where the Patient Engagement Quality Guidance (PEQG) comes in. This is the first global initiative of standardizing qualitative patient engagement (PE), building on all existing initiatives, frameworks, literature and available best practices. Last year the PEQG was launched, with a resounding positive response from all stakeholders in the healthcare ecosystem. It is a practical guide to planning, developing and assessing the quality of patient engagement activities and projects throughout the development and lifecycle of medicines. Its success can be traced back to its unique origins, which involved a diligent process of co-creation. Wide-ranging insight and expertise was sought, and the tool was ultimately co-developed thanks to the input from a large community of stakeholders (more than 100 people from 51 organizations), representing patient associations, industry, academics, researchers and external experts.
The PEQG is widely applicable, being suitable for all stakeholders working on patient engagement projects, for example, patient advocates, clinical investigators, researchers, patient organizations engaging patients or sponsors, and it is suitable for all projects. It can be used for a PE project that takes place at any point along the research and medicines-development continuum, the lifecycle of medicines, or care continuum and can be applied to health or social care research.
So how does it work? The guidance introduces seven quality criteria to assess patient engagement practices. These criteria describe the fundamental core values for a good PE practice:
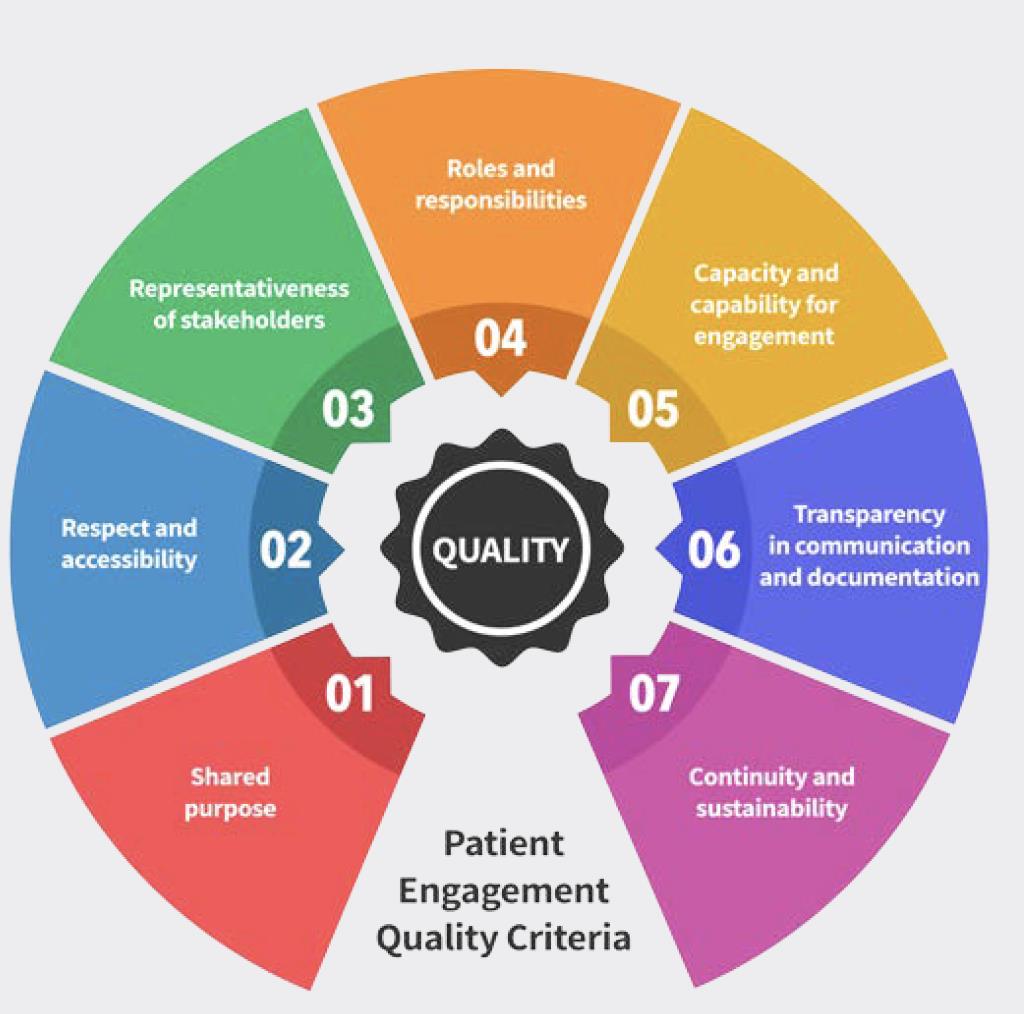
- Shared Purpose: This refers to the project’s aims and outcomes that all stakeholders taking part should agree on before starting the project.
- Respect and accessibility: This refers to (1) respecting each other, and respectful interactions within the project to be established among partners, and (2) openness and inclusion of individuals and communities (to the project) without discrimination.
- Representativeness of stakeholders: the people you involve should reflect the needs of the project and the interests of those who may benefit from project outputs (for example, target population). Consider diversity in expertise, experience, demographics, and other relevant criteria for inclusion.
- Roles and responsibilities: need for clearly agreed and ideally co-created roles and responsibilities.
- Capacity and capability for engagement: having relevant and dedicated resources from all stakeholders (for example, providing a dedicated point of contact by the sponsor and having allocated sufficient time by all stakeholders to allow genuine engagement). All stakeholders must enable meaningful engagement (for example, the level of knowledge, expertise and training stakeholders might need to deliver PE activities throughout the project).
- Transparency in communication and documentation: establishment of a communications plan and ongoing project documentation that can be shared with stakeholders. Communication among stakeholders must be open, honest and complete.
- Continuity and sustainability: Finally, this refers to the smooth progression of the project, as well as to the efforts to maintain an ongoing relationship with stakeholders.
Two versions of the PEQG Tool exist for your particular scenario – scenario 1 will help you in planning a PE project, and scenario 2 will help you assess your ongoing or completed project.
“The Guidance is the result of the close collaboration between all parties interested in achieving the best outcomes for patients when it comes to clinical research”, says The Synergist Founder and Executive Director, Nicholas Brooke. “These seven criteria describe the core values fundamental to good PE practice,” he explains, adding that a piloting scheme is now underway, with very promising reports from its implementation in a wide range of patient engagement projects. “The PEQG is a robust tool that is driving progress towards effective PE. By working with patients, we can obtain better outcomes for patients and all of us.”

Nicholas Brooke also stated that the PEQG is the foundation for further work that will see specific ‘how-to’ modules and developed training programs, focusing on specific skills, phases of development, and more than 150 patient engagement tasks such as designing clinical trial protocols with patients and job functions. “PFMD, PARADIGM and others are working on these ‘how to’ modules as we speak,” he said. Brooke added: “The rare disease patient and caregiver community all together is a pretty active and usually willing and capable to engage. The value of working with patients in the right way is therefore pretty strong and accessible for those who experience it and we are happy to support this through the PEQG and Share4Rare.”
Share4Rare aims to improve the quality of life of those affected by a rare disease. The platform bridges the gaps between the rare diseases community by connecting the researchers and disease sufferers to advance research and knowledge sharing and the Patient Engagement Quality Guidance can serve as a useful tool to achieve that.
Learn more about the Patient Engagement Quality Guidance and help us spread the word about rare diseases by sharing and joining the Share4Rare community!
