
First Consensus Meeting of the Melanoma Patient Network Europe: What does a uveal melanoma guideline have to cover?

First, what actually is uveal melanoma?
‘Uveal melanoma is a cancer that starts in the pigment-producing cells of the eye. It is one of the rare forms of melanoma as it only affects about 6 people out of 1,000,000. On very rare occasions even children can get uveal melanoma. The tumors in the eye are usually treated with radiotherapy and/or surgery but there is currently no truly effective treatment for when the disease has spread, so there is still a lot of research needed.‘
So why are you having a meeting on guidelines and who will be there?
‘Guidelines are very important as they set evidence-based standards for patient care, so they set a standard for what every patient should be receiving. We see large differences in how patients with uveal melanoma are treated across Europe — that is not acceptable. Right now, several groups are working on drafting guidelines for uveal melanoma, and this time we want to make sure that everything that matters to patients is covered!! We are a group of experienced melanoma patient advocates and all of us are somehow affected by it, either uveal melanoma and a few by the more common cutaneous melanoma. Some of us are patients, others have partners with melanoma, others have lost someone to the disease — in any case, if anyone knows what the disease does to people, it’s people like us!’
And how do you know what matters to European patients with uveal melanoma?
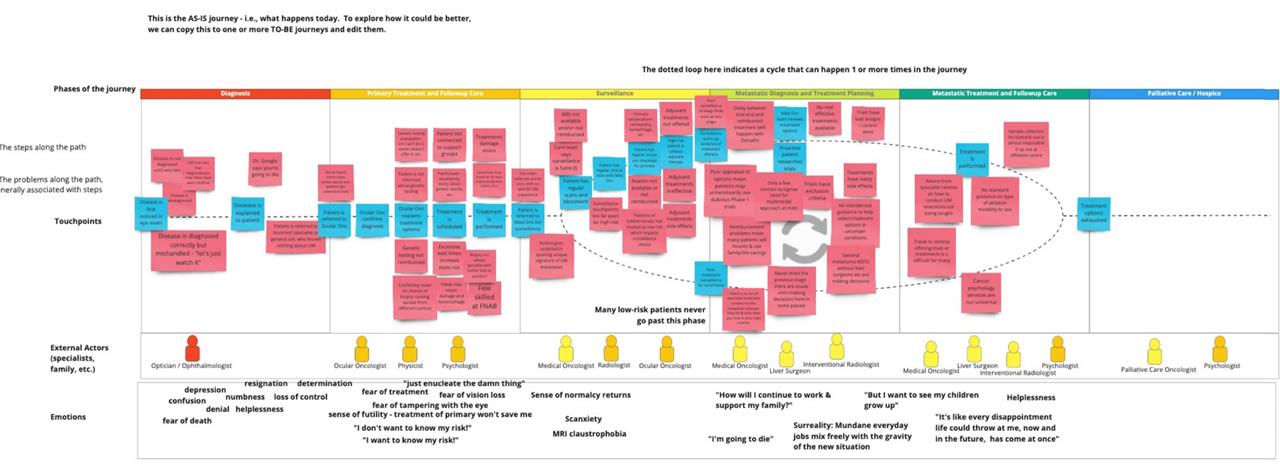
‘We started a while ago to be systematic about this. One of our colleagues developed a patient pathway map and we have been collecting the issues that uveal melanoma patients face and trying to understand them in depth. And we have done that on a European level but also for individual countries as problems can be very local.‘
So what do you hope to achieve with your Consensus Meeting?
‘The aim of the Consensus Meeting is for us to draft a list of all the areas where we recognize problems for uveal melanoma patients — and those we obviously want to see addressed in any upcoming guideline. On the top of my head, I can already tell you that the surveillance for spread of the disease and access to treatment options for patients with metastatic disease will be on that list! We also know that having a guideline, even a great one, is not enough if that guideline doesn’t get implemented everywhere, so we’ll be working on that, too. And obviously on what we as advocates need to do to make sure all European uveal melanoma patients receive the best possible treatment!’
Find out more about MPNE and their Consensus Meeting here.
The importance of clinical guidelines
In a rare disease there is often very little clinical evidence, especially when the disease becomes critical, and that which exists is limited to data from small trials, which rarely provide enough evidence to allow the compilation of National Guidelines or confirmed clinical practice. Guidelines, when followed, are useful to ensure that ALL patients receive the optimum treatment strategy despite the expertise of their treating teams or their access to appropriate therapies.
Clinical Practice Guidelines are documents that include recommendations intended to optimize patient care, which are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options. With rare diseases there is often too little evidence to review, which means there are either NO guidelines or they are not reliable and, therefore, not used. This is not helpful to patients who then receive only the basics of what standard therapies will allow, which is rarely optimal, and often not in their best interest.
Without guidelines and in order to find the best options for survival, an individual patient has to gather inaccessible evidence, try to make sense of what is usually incomparable data, and then make a decision about their own treatment pathway based on their own risk/benefit criteria. Once this almost impossible decision is made, it is normally then thwarted by what treatment strategies are available locally. Since most innovation takes years to be approved with the small patient numbers in rare disease, access to a preferred strategy is often limited to those who can afford impossibly high off-label prices or to access it overseas, which also has terrible financial and social costs.
Why a MPNE Consensus Meeting?
As a patient group who is NOT prepared to accept inequality, and who wants the best available options for all patients, the first step is to define what “good treatment” looks like for patients. Working with senior advocates from across Europe, MPNE will be gathering next week to spend 2 days working together and we will begin with a review of the problems uveal patients encounter.
What we expect after this meeting
While consensus building happens continuously on our forums in an informal way, this is the first time we will formalize the process to establish community wide actions with European patient expert advocates. Participants are selected according to specific criteria including their knowledge of published data around uveal melanoma, contributions to HTA process/clinical guidelines, detailed knowledge of the patient pathways in their countries and management/moderation of patient forums specific to uveal melanoma.
The aims of our MPNE Consensus Meeting are:
-
A guidance document that ensures that all uveal guidelines cover those areas that are currently problematic for uveal melanoma patients
-
Recommendations that will help with the implementation of guidelines in all countries
-
A companion advocacy strategy for the MPNE network that ensures that patients and advocates have access to the necessary education and tools to be involved in guidelines
