CPMS Platform for European Reference Networks (ERNs)

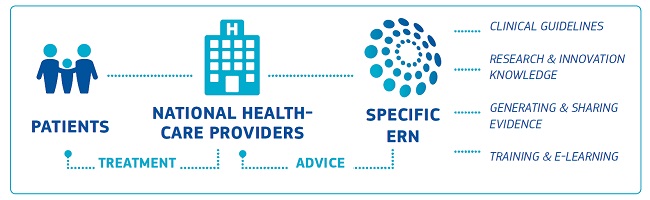
European Reference Networks (ERNs) are virtual networks involving healthcare providers across Europe who aim to tackle complex or rare diseases and conditions. There are 24 ERNs involving 25 European countries and over 300 hospitals covering all major disease groups. Each ERN has access to the CPMS (Clinical Patient Management System) to upload patient cases and to treat various diseases.
The CPMS is an IT Platform for clinical consultations between ERN members. It aims to provide the diagnosis and treatment of rare or low prevalence diseases across national borders of Member States in Europe. The platform allows healthcare professionals to collaborate virtually with one another by forming panels with other clinicians and working together to submit information and comment on findings to gain an insight into different diseases. ERN EURO-NMD uses the CPMS to assist in diagnosing and treating neuromuscular diseases.
The system is supported by a variety of different features such as medical image viewers, a family history pedigree tool and a meeting scheduler. This allows healthcare professionals to collaborate virtually so communication is quicker and more secure. The CPMS regularly undergoes updates to add new medical and administrative features so the majority of applications can be accessed under one platform whilst maintaining a user-friendly environment.
There are different user roles that each healthcare professional can request access to. The most commonly requested is the HP role, which allows a user to enrol patients, participate in panels and access meetings. The CPMS is also a useful source for researchers as they can access anonymised data via a research database on the CPMS.
Patient care and consent is incredibly important and using the CPMS maximises the security of patient data whilst discussing possible treatments, diagnosis and care. In addition to a consensus for care, patients are asked if they consent to de-identified data being included in the ERN database or registry and if they would like to be contacted about research. Consent is a legal requirement in line with GDPR (General Data Protection Regulation) and patients have a right to access data held about them.
Overall, the CPMS will make diagnosis and treatment more efficient since expert clinicians can collaborate virtually on cases. It will be a less stressful experience for the patient as it will mean that there will be no need for them to travel. Since all information is handled on a secure platform, patients can also be reassured that their data is protected.
European Patient Advocacy Groups (ePAGs) have been developed by EURODIS for each ERN disease group so patient organisations are able to participate in ERN decision making. ePAGS will bring together elected patient representatives and affiliated organisations to ensure that the patient voice can be heard throughout the ERN development process. ePAG patient representatives have an important role of representing the wider patient community in the development of ERNs by participating In Board and sub-clinical committees within their respective ERN. By encouraging patient organisations to participate in decisions it means that the patient voice will always be heard and patients will be represented within their ERN.
There are currently 24 ERNs covering various disease groups:
|
European Reference Network |
Disease Group |
Website |
|
ERN BOND |
Bone disorders |
|
|
ERN CRANIO |
Craniofacial anomalies and ear, nose and throat (ENT) disorders |
|
|
Endo-ERN |
Endocrine conditions |
|
|
ERN EpiCARE |
Epilepsies |
|
|
ERKNet |
Kidney diseases |
|
|
ERN-RND |
Neurological diseases |
|
|
ERNICA |
Inherited and congenital anomalies |
|
|
ERN LUNG |
Respiratory diseases |
|
|
ERN Skin |
Skin disorders |
|
|
ERN EURACAN |
Adult cancers (solid tumours) |
|
|
ERN EuroBloodNet |
Haematological diseases |
|
|
ERN eUROGEN |
Urogenital diseases and conditions |
|
|
ERN EURO-NMD |
Neuromuscular diseases |
|
|
ERN EYE |
Eye diseases |
|
|
ERN GENTURIS |
Genetic tumour risk syndromes |
|
|
ERN GUARD-HEART |
Diseases of the heart |
|
|
ERN ITHACA |
Congenital malformations and rare intellectual disability |
|
|
MetabERN |
Hereditary metabolic disorders |
|
|
ERN PaedCan |
Paediatric cancer (haemato-oncology) |
|
|
ERN RARE-LIVER |
Hepatological diseases |
|
|
ERN ReCONNET |
Connective tissue and musculoskeletal diseases |
|
|
ERN RITA |
Immunodeficiency, auto inflammatory and autoimmune diseases |
|
|
ERN TRANSPLANT-CHILD |
Transplantation in Children |
|
|
VASCERN |
Rare Multisystemic Vascular Diseases |
